Abstract
Introduction: Multiple myeloma (MM) is a chronic hematologic malignancy with a high symptom burden that can have a substantial negative impact on patients' (pts) health-related quality of life (HRQoL). The introduction of novel triplet regimens for newly diagnosed MM (NDMM) has extended progression-free survival (PFS) and overall survival (OS); however, adverse events and demanding administration and monitoring schedules have a further negative effect on HRQoL, especially among pts who are transplant ineligible (TIE) due to older age and/or frailty. Optimizing initial treatment is particularly important in older pts, many of whom receive only 1 line of therapy. The phase 3 MAIA trial compared daratumumab, lenalidomide, and dexamethasone (D-Rd) vs lenalidomide and dexamethasone (Rd) in TIE pts with NDMM. At a median follow-up of 28 months, D-Rd significantly prolonged PFS and was associated with faster and sustained clinically meaningful improvements in patient-reported outcomes (PROs) vs Rd. Results of an updated analysis with longer follow-up recently confirmed a significant benefit in OS with D-Rd vs Rd as well as a continued significant PFS benefit and higher rates of complete response or better and very good partial response or better. Here we present an update of the HRQoL analysis with additional follow-up.
Methods: MAIA (NCT02252172) is a randomized, open-label, active controlled, multicenter, phase 3 study of TIE pts with NDMM who were randomly assigned 1:1 to receive D-Rd or Rd until disease progression (PD) or unacceptable toxicity. PROs were recorded using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30-item (EORTC QLQ-C30) and the EQ-5D-5L visual analog scale. EORTC QLQ-C30 has 30 items comprising 5 functional scales (physical, role, emotional, cognitive, and social functioning), 1 global health status (GHS) scale, 3 symptom scales (fatigue, nausea and vomiting, and pain) and 6 single items (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). Questionnaires were completed at baseline, on day 1 of cycles 3, 6, 9, and 12 for year 1, and every 6 months thereafter until PD. Analyses were conducted on all pts with a baseline and ≥1 post-baseline PRO assessment. Pts were censored at PD or discontinuation of study treatment. Thresholds for meaningful improvement and worsening were defined a priori based on published literature (≥10-point change). Treatment effect was analyzed using a mixed-effects model for repeated measurements including baseline value, visit, treatment, visit by treatment interaction, and randomization stratification factors as fixed effects and individual subject as random effect.
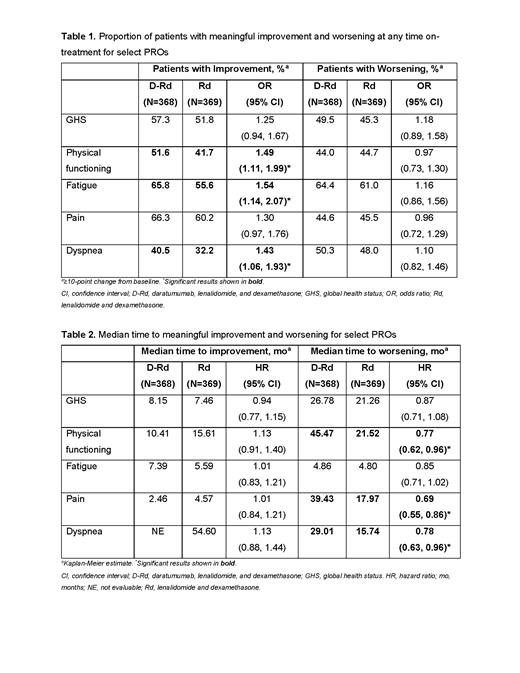
Results: At a median follow-up of 56.2 months, discontinuation rates were lower with D-Rd than Rd (56.8% vs 80.8%). For GHS, physical functioning, fatigue, pain, and dyspnea, PROs that are particularly relevant to pts with MM, a numerically greater proportion of pts achieved a meaningful improvement with D-Rd vs Rd (Table 1). Differences were significant for physical functioning, fatigue, and dyspnea. The proportion of pts achieving a meaningful worsening on therapy was similar in both treatment groups (Table 1). The median time to improvement was numerically shorter with D-Rd vs Rd for physical functioning and pain and with Rd vs D-Rd for GHS and fatigue; differences were not significant (Table 2). The median time to worsening of fatigue was similar between groups, numerically longer for D-Rd vs Rd for GHS, and significantly longer with D-Rd than Rd for physical functioning, pain, and dyspnea (Table 2). Median time to worsening of pain with D-Rd vs Rd was 39.43 vs 17.97 months, reflective of an additional ~21 months without worsening pain among pts treated with D-Rd. Between-group differences for least squares mean change from baseline for these 5 PROs favored D-Rd vs Rd at all assessment time points except cycle 3 for physical functioning and cycle 6 for fatigue; differences were significant at ≥1 timepoint for each scale.
Conclusions: These updated PRO analyses from the MAIA study demonstrate sustained and clinically meaningful improvements in HRQoL with D-Rd vs Rd with almost 5 years of follow-up. These results are consistent with the clinical benefits of superior PFS, OS, and deep responses observed with D-Rd compared with Rd and support the use of D-Rd in older pts.
Perrot: Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kumar: Roche-Genentech: Consultancy, Research Funding; Novartis: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding; Bluebird Bio: Consultancy; Tenebio: Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Carsgen: Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Antengene: Consultancy, Honoraria; Oncopeptides: Consultancy; Beigene: Consultancy; BMS: Consultancy, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Plesner: Takeda: Research Funding; Oncopeptides: Other: Advisor, Research Funding; Genentech: Other: Advisor, Research Funding; CSL Behring: Other: Advisor; AbbVie: Other: Advisor, Research Funding; Celgene: Other: Advisor, Research Funding; Janssen: Other: Advisor, Research Funding; Genmab: Research Funding. Orlowski: Amgen, Inc., BioTheryX, Inc., Bristol-Myers Squibb, Celgene, EcoR1 Capital LLC, Genzyme, GSK Biologicals, Janssen Biotech, Karyopharm Therapeutics, Inc., Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda P: Consultancy, Honoraria; CARsgen Therapeutics, Celgene, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Other: Clinical research funding; Asylia Therapeutics, Inc., BioTheryX, Inc., and Heidelberg Pharma, AG.: Other: Laboratory research funding; Asylia Therapeutics, Inc.: Current holder of individual stocks in a privately-held company, Patents & Royalties; Amgen, Inc., BioTheryX, Inc., Bristol-Myers Squibb, Celgene, Forma Therapeutics, Genzyme, GSK Biologicals, Janssen Biotech, Juno Therapeutics, Karyopharm Therapeutics, Inc., Kite Pharma, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, I: Membership on an entity's Board of Directors or advisory committees. Moreau: Oncopeptides: Honoraria; Sanofi: Honoraria; Janssen: Honoraria; Celgene BMS: Honoraria; Amgen: Honoraria; Abbvie: Honoraria. Bahlis: BMS/Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Genentech: Consultancy; GlaxoSmithKline: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria. Nahi: XNK Therapeutics AB: Consultancy. Hulin: Takeda: Honoraria; Sanofi: Honoraria; Celgene/BMS: Honoraria; Janssen: Honoraria; abbvie: Honoraria. Quach: CSL: Consultancy, Membership on an entity's Board of Directors or advisory committees; Antengene: Consultancy, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen/Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees. Goldschmidt: Chugai: Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; BMS: Consultancy, Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; Incyte: Research Funding; Janssen: Consultancy, Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; Johns Hopkins University: Other: Grant; Molecular Partners: Research Funding; MSD: Research Funding; Mundipharma: Research Funding; Sanofi: Consultancy, Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; Takeda: Consultancy, Research Funding; Adaptive Biotechnology: Consultancy; Celgene: Consultancy, Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; Novartis: Honoraria, Research Funding; Dietmar-Hopp-Foundation: Other: Grant; GSK: Honoraria; Amgen: Consultancy, Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding. O'Dwyer: ONK Therapeutics: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; Bristol Myers Squibb: Research Funding. Venner: BMS: Honoraria; Amgen: Research Funding; Celgene: Research Funding; Amgen: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Sanofi: Honoraria; Pfizer: Honoraria. Weisel: Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; Novartis: Honoraria; Pfizer: Honoraria. Raje: Celgene, Amgen, Bluebird Bio, Janssen, Caribou, and BMS: Other. Macro: Sanofi: Honoraria; GSK: Honoraria; Takeda: Honoraria, Other: Travel accomodation, Research Funding; Janssen: Honoraria, Other: Travel accomodation, Research Funding; Celgen/BMS: Honoraria. Leleu: Sanofi: Honoraria; Takeda: Honoraria, Other: Non-financial support; Janssen-Cilag: Honoraria; Karyopharm Therapeutics: Honoraria; Merck: Honoraria; Mundipharma: Honoraria; Novartis: Honoraria; Oncopeptides: Honoraria; Gilead Sciences: Honoraria; Celgene: Honoraria; Carsgen Therapeutics Ltd: Honoraria; Bristol-Myers Squibb: Honoraria; Amgen: Honoraria; AbbVie: Honoraria; Pierre Fabre: Honoraria; Roche: Honoraria. Liu: Janssen: Current Employment, Current equity holder in publicly-traded company. Fastenau: Janssen: Current Employment, Current equity holder in publicly-traded company. Gries: Janssen: Current Employment, Current holder of individual stocks in a privately-held company. Ho: DRG Abacus: Consultancy; Janssen: Consultancy; Emalex Biosciences: Consultancy. Mistry: Janssen: Current Employment, Current equity holder in publicly-traded company. Tromp: Janssen: Current Employment, Current equity holder in publicly-traded company. Delioukina: Janssen: Current Employment. Vermeulen: Janssen: Current Employment, Current equity holder in publicly-traded company. Usmani: Abbvie: Consultancy; Array BioPharma: Consultancy, Research Funding; Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; GSK: Consultancy, Research Funding; EdoPharma: Consultancy; Janssen: Consultancy, Research Funding, Speakers Bureau; Sanofi: Consultancy, Research Funding, Speakers Bureau; Merck: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; SkylineDX: Consultancy, Research Funding; Takeda: Consultancy, Research Funding, Speakers Bureau; Janssen Oncology: Consultancy, Research Funding; Bristol-Myers Squibb: Research Funding; Amgen: Consultancy, Research Funding, Speakers Bureau.